Sarepta’s Commitment to SRP-9001 Approval Undeterred by Recent AdCom Setback
April 21, 2023
Trending News ☀️
Sarepta Therapeutics ($NASDAQ:SRPT) is a biotechnology company that focuses on the development and commercialization of precision genetic medicines to treat rare and infectious diseases. The company recently experienced a setback from the US Food and Drug Administration’s (FDA) AdCom, when it voted to reject its application for approval of SRP-9001 for the treatment of Duchenne Muscular Dystrophy (DMD). Despite this setback, Sarepta remains undeterred in its commitment to secure approval for SRP-9001. The AdCom’s negative vote was mainly due to the lack of clinical data for SRP-9001, which is an experimental drug.
However, Sarepta remains confident that the FDA will ultimately approve the drug. The FDA has granted Breakthrough Therapy Designation (BTD) to SRP-9001, indicating that it recognizes the drug’s potential to address an unmet medical need.
In addition, the company has presented additional data on the efficacy of SRP-9001 to the FDA. These data points suggest that the drug is effective and that the benefit-risk profile of SRP-9001 is favorable. Given the compelling evidence, Sarepta is optimistic that it will overcome this setback and gain approval for SRP-9001. If approved, SRP-9001 would be the first and only US approved treatment for DMD, which would be a major breakthrough for patients and families suffering from this debilitating condition. As such, Sarepta’s commitment to secure approval for SRP-9001 remains strong.
Price History
Sarepta Therapeutics Inc. recently experienced a setback in the approval of its drug, SRP-9001, as the U.S. Food and Drug Administration’s (FDA) advisory committee voted not to support the approval. Despite this challenge, Sarepta is still dedicated to obtaining approval for SRP-9001. On Wednesday, Sarepta’s stock opened at $125.0 and closed at $124.1, down by 1.1% from its previous closing price of $125.6. Until then, Sarepta will continue to work towards securing approval for SRP-9001, in order to meet the needs of their patients. Live Quote…
About the Company
Income Snapshot
Below shows the total revenue, net income and net margin for Sarepta Therapeutics. More…
| Total Revenues | Net Income | Net Margin |
| 933.01 | -703.49 | -67.6% |
Cash Flow Snapshot
Below shows the cash from operations, investing and financing for Sarepta Therapeutics. More…
| Operations | Investing | Financing |
| -325.35 | -1.05k | 232.51 |
Balance Sheet Snapshot
Below shows the total assets, liabilities and book value per share for Sarepta Therapeutics. More…
| Total Assets | Total Liabilities | Book Value Per Share |
| 3.13k | 2.74k | 4.38 |
Key Ratios Snapshot
Some of the financial key ratios for Sarepta Therapeutics are shown below. More…
| 3Y Rev Growth | 3Y Operating Profit Growth | Operating Margin |
| 34.8% | – | -68.2% |
| FCF Margin | ROE | ROA |
| -38.2% | -97.6% | -12.7% |
Analysis
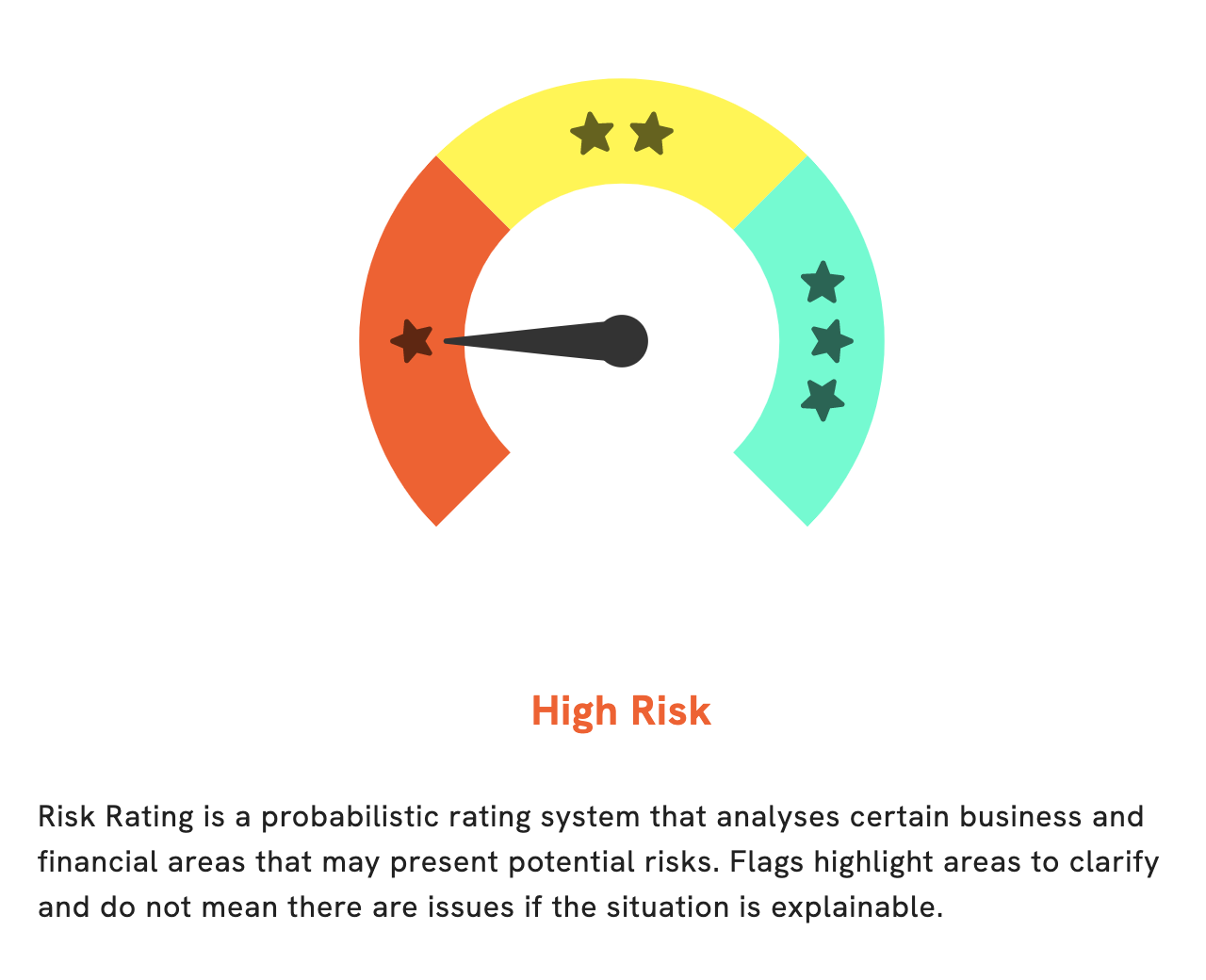
At GoodWhale, we recently conducted an analysis of SAREPTA THERAPEUTICS and based on our Risk Rating algorithm, it is a high risk investment in terms of financial and business aspects. Upon further inspection, we’ve detected 4 risk warnings in the different financial documents including the income sheet, balance sheet, cash flow statement, and financial journal. If you’re looking for more information about the risks of investing in SAREPTA THERAPEUTICS, please register on our website and check out the details for yourself. More…
Peers
The company is headquartered in Cambridge, Massachusetts and was founded in 1980. Sarepta Therapeutics Inc has four main competitors: Genor Biopharma Holdings Ltd, Impel Pharmaceuticals Inc, Entera Bio Ltd, and PTC Therapeutics Inc. These companies are all focused on the development of treatments for DMD and other rare diseases.
– Genor Biopharma Holdings Ltd ($SEHK:06998)
Genor Biopharma Holdings Ltd is a pharmaceutical company that focuses on the development and commercialization of innovative drugs for the treatment of cancer. The company has a market cap of 949.11M as of 2022 and a ROE of -23.99%. The company’s products are designed to target specific genetic mutations that are known to drive the growth and progression of cancer.
– Impel Pharmaceuticals Inc ($NASDAQ:IMPL)
Impel Pharmaceuticals Inc is a pharmaceutical company with a market cap of 94.72M as of 2022. The company has a Return on Equity of -359.89%. Impel Pharmaceuticals Inc is a company that focuses on the development and commercialization of drugs for the treatment of central nervous system disorders.
– Entera Bio Ltd ($NASDAQ:ENTX)
Entera Bio Ltd is a clinical-stage biopharmaceutical company focused on the development and commercialization of oral therapeutics for serious unmet medical needs. The company’s lead product candidate is EB614, an oral biologic that is in clinical development for the treatment of osteoporosis, inflammatory bowel disease, and other immune-mediated diseases. Entera Bio Ltd has a market cap of 24.06M as of 2022, a Return on Equity of -48.6%. The company’s focus on the development and commercialization of oral therapeutics makes it a unique player in the biopharmaceutical market. However, its negative ROE indicates that it is not a profitable company at this time.
Summary
Sarepta Therapeutics Inc. is a biotechnology company focused on developing treatments for rare neuromuscular diseases. Despite a recent setback from the AdCom, Sarepta’s prospects for approval of SRP-9001 remain strong. Analysts point to the strength of the Phase 3 data, patient testimonials, and the company’s track record of success in bringing therapies to market. Investors should be encouraged by the FDA’s statements about the efficacy of SRP-9001, as well as the lack of competition in the space.
Furthermore, recent acquisitions and partnerships could provide additional revenue streams for investors. Sarepta’s stock price remains attractive, and investors should continue to monitor the situation closely.
Recent Posts